What Bioengineering Tools?
1. Biomaterials
2. Transgenic Cells
3. Viral Vectors
BIOMATERIALS
Biomaterials are the foundation of our bioengineering toolkit, and we incorporate custom-synthesized biomaterials across all our research areas. Our newly synthesized biomaterials afford emergent or enhanced physiochemical/biological properties compared to existing material options.
Our Overarching Approach: We have a unique expertise in synthesizing new bioactive polymers, which we achieve by combining innovative enzymatic catalyzed monomer synthesis with modern chemistry techniques including living polymerization methods, thiol-ene click reactions, and thioether chemistry.
We currently formulate these bioactive polymers into 3 different biomaterial platforms using biologically inspired supramolecular assembly:
(1) Hydrogels
(2) Coacervates
(3) Coatings/Films
We use biomaterials in a variety of ways to manipulate the functions of glia and the cells they interact with, including as: (i) carriers for cell grafts or bioactive molecules, (ii) intracellular drug delivery vehicles, and (iii) substrates to regulate device-tissue interactions.
Keep reading for more details about our different biomaterial platforms…
Hydrogels
Our Approach: We are currently developing a variety of new supramolecular hydrogels that have unique bioactivity by using standardized approaches to synthesize new monomers and polymers. To prepare monomers from various bioactive molecules such as saccharides, nucleosides, and other metabolic intermediates, we employ a lipase catalyzed acylation reaction to endow these molecules with acrylate or methacrylate reactive groups. Using the enzymatic catalyzed reactions ensures selective addition of the acrylic reactive groups to these molecules such that their bioactivity is maintained. Polymers composed of at least two of these bioactive molecules is achieved through reacting the acrylic monomer versions of these bioactive molecules using free radical polymerization (FRP) or a specific-type of controlled living polymerization known as Reversible addition−fragmentation chain-transfer (RAFT). Using such methods we can prepare statistical or block copolymers. Many of the bioactive molecules we work with participate in specific non-covalent interactions such as hydrogen bonding, crystallization, or electrostatic interactions. Leveraging these non-covalent interactions we can induce copolymers to self-assemble into long-range ordered physical hydrogels via interactions between individual polymer chains or by encourage polymer chain interactions with specific small molecule gelation inducing agents. By tailoring the composition of bioactive molecules used, we formulate locally injectable hydrogels to specifically manipulate key glial cell functions to enhance glia-based repair at CNS injuries.
An overview of our hydrogel platform:
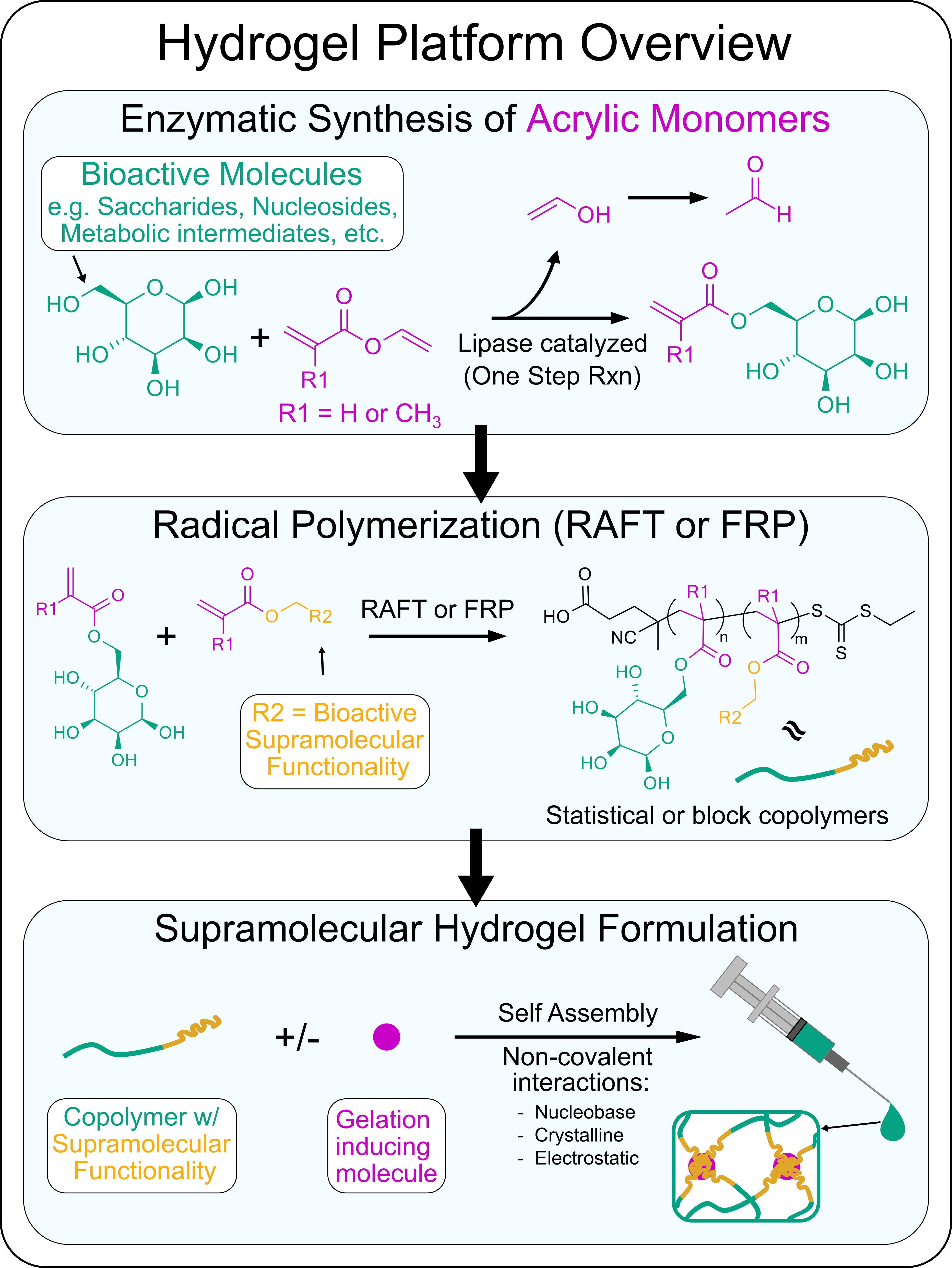
Example: A recently developed hydrogel system from our lab that uses the approach outlined above involved using newly synthesized poly(trehalose-co-guanosine) (pTreGuo) glycopolymers and formulating pTreGuo with free guanosine (fGuo) to generate shear-thinning hydrogels through stabilized long-range G-quadruplex secondary structures. Injections of pTreGuo-based hydrogels into ischemic stroke injuries altered the natural responses of glial cells after injury to reduce the size of lesions and increase axon regrowth into lesion core environments (DuBois et al. 2023. Advanced Materials).
Coacervates
Our Approach: We are currently developing a variety of different coacervate formulations for use as drug delivery vehicles and cell graft carriers. Coacervates are polymer-rich liquid droplets formed by liquid-liquid phase separation that is induced upon ionic complexation of two oppositely charged polyelectrolytes. Coacervates can be formulated to cross the cell membrane thus affording intracellular delivery of cargo and the coacervate carrier, or they can also be tailored to remain extracellularly and coalesce into a bulk phase with tunable mechanical properties that can be used to suspend and disperse therapeutics into discrete neural tissues beds via localized injection. Coacervates permit high concentration loading and controlled release of bioactive growth factors, enzymes, and antibodies, with modular formulation parameters enabling tunable release kinetics.
To prepare coacervates we combine enzymatic catalyzed and thiol-ene click reactions to generate multivalent polyelectrolytes (polyanionic and polycationic) that are based on branched polymer or dendrimer structures. Mixing of oppositely charged multivalent polyelectrolytes under mild, physiologically buffered conditions results in complex coacervate formation.
An overview of our coacervate platform:
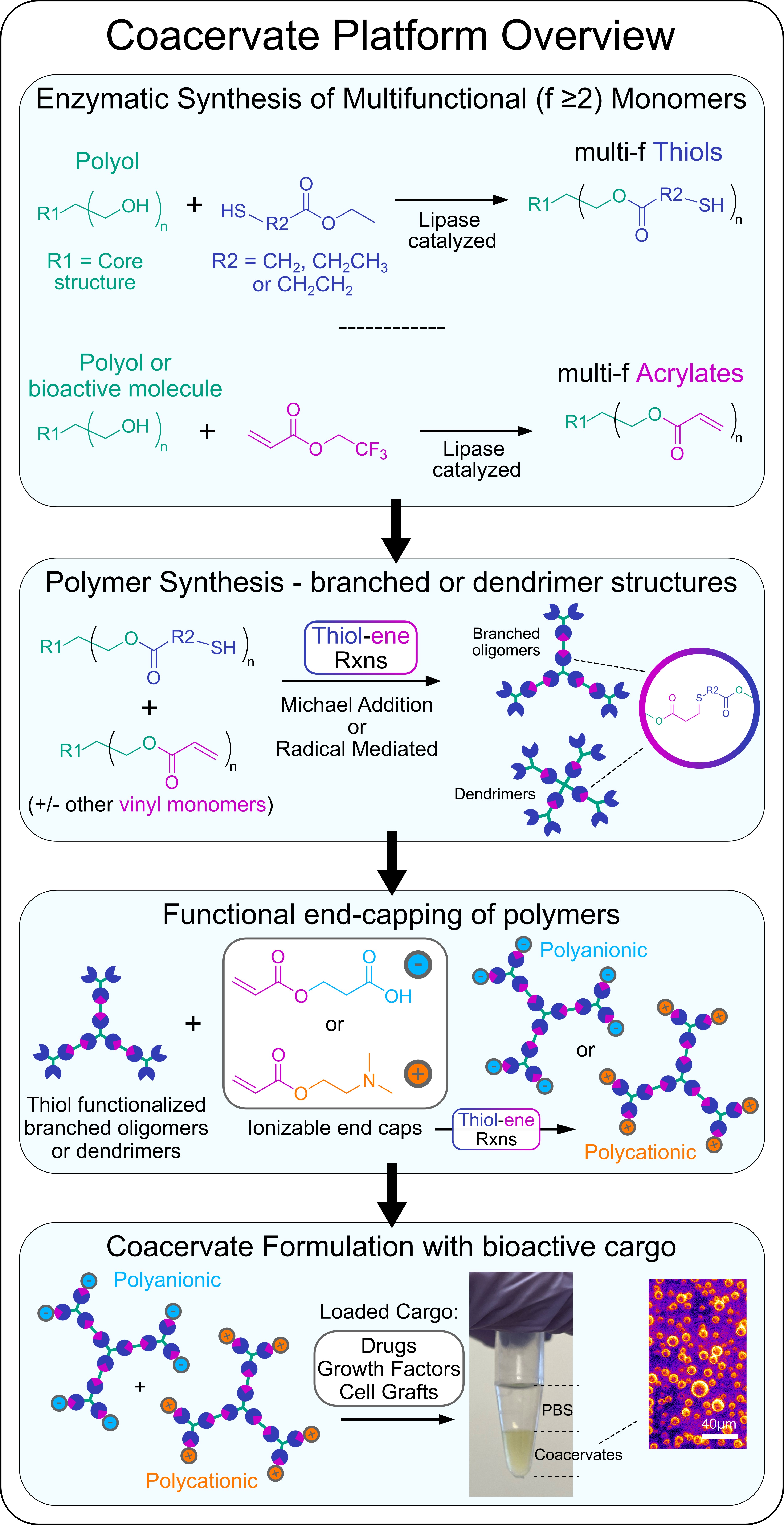
Example: A recently developed coacervate system from our lab (Hassan et al. 2024. Biomaterials) involves the use of biodegradable trehalose-based polyelectrolyte oligomers that are prepared using using enzymatic catalyzed monomer synthesis and facile A2:B3:AR thiol-ene Michael addition reactions. Complex coacervates form upon mixing of oppositely charged trehalose-based oligomers. Trehalose is a non-reducing disaccharide that is produced by yeast and other extremophile organisms to effectively stabilize intracellular proteins, nucleic acids, and membranes to enhance survival of their constituent cells under extreme environments. The unique strength, spatial orientation, and symmetry of hydrogen bonding afforded by trehalose has also conferred enhanced thermal and mechanical stabilization of biologic therapeutics as a formulation excipient. Trehalose coacervates leverage the stabilizing functionality of the constituent disaccharide excipient while affording new coacervate-specific properties including precisely delivering biomolecules to the CNS in vivo that we are using to enhance glia-based repair at CNS injuries and prepare improved cell graft biomaterial carriers.
COATings/FILMS
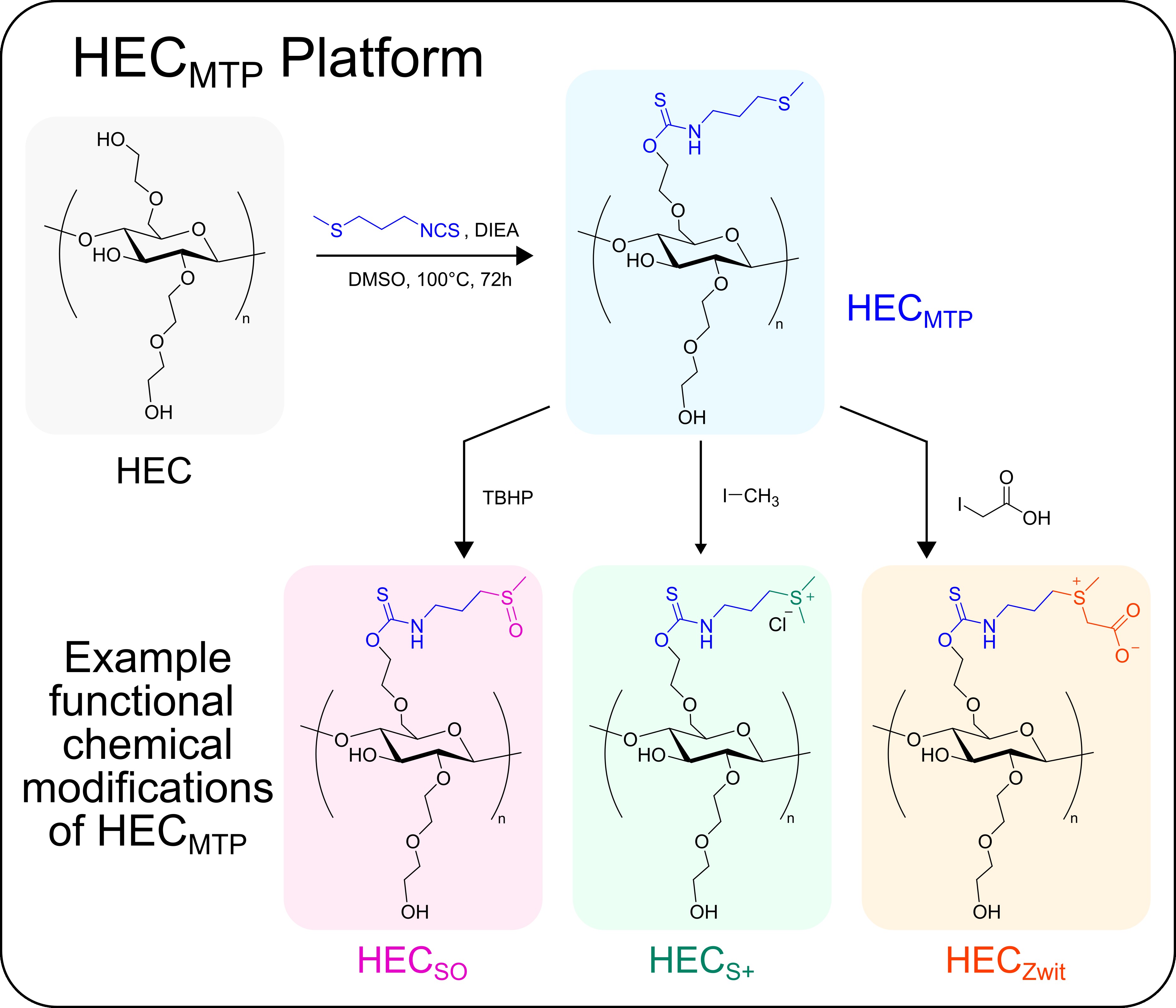
Our Approach: We are currently developing new modular cellulose-based polymers that can be formulated as non-resorbable coatings or films for use in optical and in vivo drug delivery applications in the brain as well as for fundamental studies of biomaterial-tissue interactions and foreign body responses (FBR).
An overview of our coatings/films platform:

Example: Recently, we applied the above outlined approach to modify hydroxyethyl cellulose (HEC) with thioether groups to generate an oxidation-responsive polymer, HECMTP (Dubois et al. 2024. Advanced Healthcare Materials). HECMTP can be readily formulated into coatings or films, is non-resorbable and biocompatible in vivo, and permits oxidation driven changes in the polymer chemistry under physiological conditions that contributes to increased wettability and decreased stiffness of a coated substrate without evoking bulk swelling or delamination of the polymer from that underlying substrate. We are actively exploring the use of HECMTP and other variants (see below) to improve the long-term biocompatibility of medical devices and to endow these devices with additional functionalities and biological properties.
TRANSGENIC CELLS
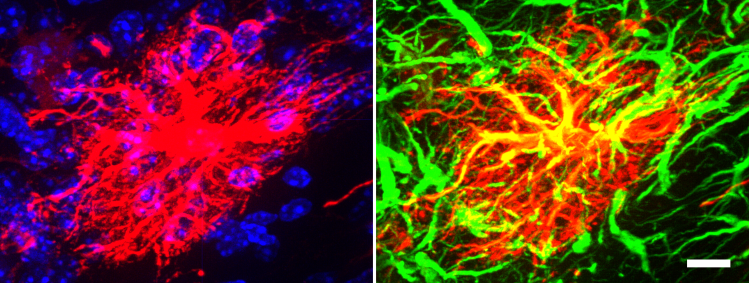
Our Approach: We use transgenic mouse embryonic stem cell (ESC) and neural progenitor cell (NPC) lines to derive glial cells for our transplantation studies and to perform in vitro cell culture investigations to dissect basic biological mechanisms as well as glia-biomaterial interactions. One such transgenic NPC line contains a RiboTag (hemagglutinin (HA) tag on ribosomal protein L22) allele to facilitate cell specific genetic evaluation of grafted cells from whole tissue in vivo as well as identification of NPC and their progeny by immunohistochemistry (using HA as a reporter). Below is a transplanted NPC (identified by HA reporter – red) that has been directed to differentiate into a mature astrocyte (GFAP – green) in vivo in the uninjured mouse brain (scale bar=10 µm).
Read more about our work using transgenic NPC lines for cell grafting into CNS injuries in the following publications:
VIRAL VECTORS
With the help of collaborators we use Adeno-associated virus (AAV) to mediate sustained expression of molecular cues in neurons, glia or non-neural lesion core cells.