RESEARCH AREAS
GLIA Engineering of:
1. Traumatic CNS InjURIES
2. Neural Interfaces
3. Drug Delivery
1. Glia Engineering of Traumatic CNS Injuries
Scientific Premise: The mammalian adult CNS fails to regenerate after injury because the parenchymal cells responsible for neural tissue function (neurons & glia) lack sufficient self-renewal capacity. However, in mammalian neonates (newborns), functional neural circuit regeneration is possible and is accomplished through proliferating immature astrocytes which direct scar-free parenchymal repair. Astrocyte-directed parenchymal repair capacity is short-lived and is completely lost after the first postnatal week of development in rodent model systems. Beyond this period, astrocytes transition into quiescent and non-migratory cells, adopting complex morphologies and functions necessary to maintain healthy neural circuit viability. Upon adult CNS injury, a spatially restricted population of locally surviving astrocytes undergo dynamic adaptive reprogramming that involves de-differentiation, alterations to metabolic programs, and re-entry into the cell cycle to lead the CNS parenchymal repair response. However, because this adult astrocyte wound repair response is delayed, spatially restricted, and transient, it only results in a narrow, protective astroglial border but not the comprehensive parenchymal repair necessary to support functional regeneration. Thus, the scientific premise guiding this research area is that augmenting the presence and functions of proliferating immature astrocytes at adult CNS injury lesions will enhance parenchymal repair capacity to provide the necessary support for neural circuit regeneration.
Why is this important?: In traumatic CNS injuries destruction of neural tissue initiates a natural wound healing response that results in lesion formation. CNS injury lesions are composed of non-neural lesion cores that are isolated from adjacent viable neural tissue by astrocyte borders. At the lesion site, there is no spontaneous regeneration of new neural tissue. Instead, the non-neural lesion core that persists chronically lacks the structural and molecular support necessary for neural circuit repair and as a consequence spontaneous or stimulated regeneration through large CNS lesions either does not occur or is not sustained long term. In the healthy CNS the structural and molecular support that ensures persistent neural circuit viability is usually provided by a diversity of glia.
Current projects: We are pursuing two projects that use innovative bioengineering strategies to augment the presence of proliferating immature astrocytes at adult CNS injuries either by directing the functions of endogenous host astrocytes or grafting cells that can provide an exogenous source of astrocytes.
Project #1: Activating endogenous glia repair mechanisms in adult astrocytes in spinal cord injury (SCI)

The overall objective here is to mechanistically dissect the molecular requirements for enhancing astrocyte self-renewal and migratory capacity after SCI. In this project, we are using in vitro astrocyte cell cultures and a mouse crush spinal cord injury (SCI) model to dissect and direct this astrocyte biology.
Our overall hypothesis is that increasing self-renewal and migratory capacity in adult astrocytes after SCI will require modulating natural growth factor, purinergic, and immune signaling as well as the availability of critical metabolic fuels.
A key rationale for our approach here is that synthetic injectable biomaterials can be engineered to sustain the delivery of specific molecular cues to prolong the duration and extent of immature-like functions in astrocytes.
We are actively exploring several biomaterial-based strategies to enhance astrocyte self-renewal and migratory capacity after SCI. Each initiative listed below includes biomaterials engineering activities to address unique challenges. Key initiatives within this project include:
- Simultaneously delivering pro-proliferative growth factors and small molecule inhibitors of anti-mitotic cues to overcome the natural cytostatic signaling that dominates at SCI lesion environments.
- Providing prolonged localized availability of critical metabolic fuels necessary to sustain astrocyte proliferation at SCI lesions.
- Locally delivering specific combinations of nucleosides and nucleotides to alter purinergic signaling that regulates astrocyte proliferation and chemotaxis at SCI lesions.
- Directing intracellular delivery of exogenous transcription factors to activate pro-proliferative gene expression in astrocytes at SCI lesions.
- Delivering immunoregulating molecules to overcome the natural non-cell autonomous perturbation of astrocyte functions by recruited immune cells at SCI lesions.
Project #2: Using cell grafts to direct glia repair in stroke.

Rationale: Glia repair after CNS injury can be enhanced by grafting exogenous sources of cells that are capable of generating wound repair astroglia that serve neuroprotective functions such as restricting inflammation and preventing fibrotic scarring. Graft-derived glia repair mimics the scar-free wound repair that occurs naturally in mammalian neonates that supports effective neural circuit regeneration. Enhancing glia repair actions of cell grafts in acute injuries may be a functional basis or starting point for rebuilding neural tissue at CNS injury lesion cores. However, grafts made into very severe CNS injuries show mostly ineffective glia repair, indicating that grafting outcomes are regulated non-cell autonomously in a lesion environment-dependent manner.
This project has been guided by our recent experimental observations showing that acute lesion environments created by a specific chemical stroke model, L-NIO, provides unique trophic support to grafted cells such that the survival and glia repair functions of grafted cells is superior at these lesions than at any other environment, including hemorrhagic strokes, photothrombotic strokes, and healthy neural tissue.
The current goal for this project is to identify the type and source of trophic support that underpins the favorable grafting outcomes at supportive L-NIO lesions through a systematic evaluation of host cell contributions. Using these new insights, we are testing biomaterial-based strategies to recreate key graft supportive aspects of the L-NIO lesion environments or deliver important L-NIO-like molecular cues to improve graft-derived glia repair outcomes in more clinically relevant, severe stroke models (photothrombotic ischemic stroke and collagenase-induced hemorrhagic stroke).
Another important facet of this work is the incorporation of cutting-edge intravital imaging methods to longitudinally track grafting outcomes at strokes and provide a way to evaluate graft-derived glia repair non-invasively. We are collaborating with David Boas (BU BME) and, enabled by a co-advised student, we are applying two-photon microscopy and optical coherence tomography to image graft-directed glia repair.
2. Glia Engineering of Neural Interfaces
Our Approach: Using our bioengineering tools and standardized in vivo methods we: (i) study the functions of specific CNS glial cell types in the CNS foreign body response (FBR) to biomaterials generally as well as to specific chronically implanted neural devices, (ii) evaluate material properties that dominantly regulate FBR severity, and (iii) develop biomaterial-based strategies that manipulate glia to minimize the FBR at implant systems. We hypothesize that bioengineering strategies that manipulate how glia perceive or respond to biomaterials/foreign bodies may be leveraged to develop better performing CNS implants.
Astrocyte border formation is a central feature of FBR to chronically implanted devices that cause premature device failure and is derived from adaptive reprogramming of astrocytes. How, and how many, astrocytes are reprogrammed into border states ultimately determines the extent of neural tissue disrupted around devices. Astrocyte borders are not static once formed and can be exacerbated by implant micromotion, latent infection, or persistent device-derived stimuli. Astrocyte border formation is a conserved feature of CNS FBRs across all different types of implants, but context-specific functions of astrocyte borders are poorly understood. The scientific premise for this research area is that biomaterials can be used as tools to study and regulate astrocyte border formation and functions to improve our understanding of conserved- and context-dependent features of astrocyte borders and guide the development of chemistries to minimize or evoke specific astrocyte responses.
Current Projects: An overview of the two projects we are currently working on in this research area are outlined below.
Project #1: Transcriptional and morphological profiling of astrocyte borders using injectable biomaterials.
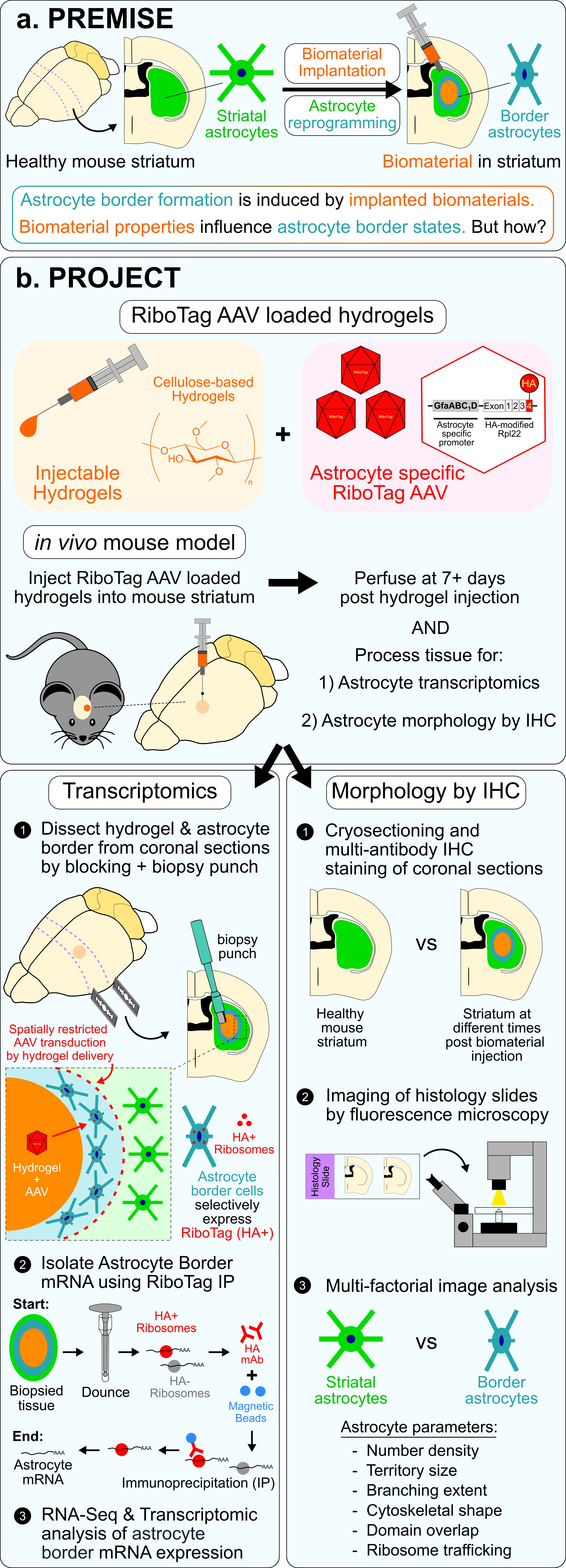
In this project we are incorporating injectable biomaterials into a new bioassay to stimulate and characterize astrocyte borders formed in the mouse striatum and use this bioassay to profile several different functional states of astrocyte borders conferred by interactions with diverse non-neural cell populations. In this project, we are using non-resorbable cellulose-based biomaterials engineered with specific chemistries as a way to alter the immune cell composition of the non-neural niche at the biomaterial-tissue interface and consequently direct distinct astrocyte borders. We are incorporating local delivery of astrocyte-specific viral vectors from biomaterials to molecularly characterize astrocyte borders by cell-specific transcriptomic methods.
Project #2: Investigating astrocyte reprogramming to improve the performance of chronically implanted neural devices
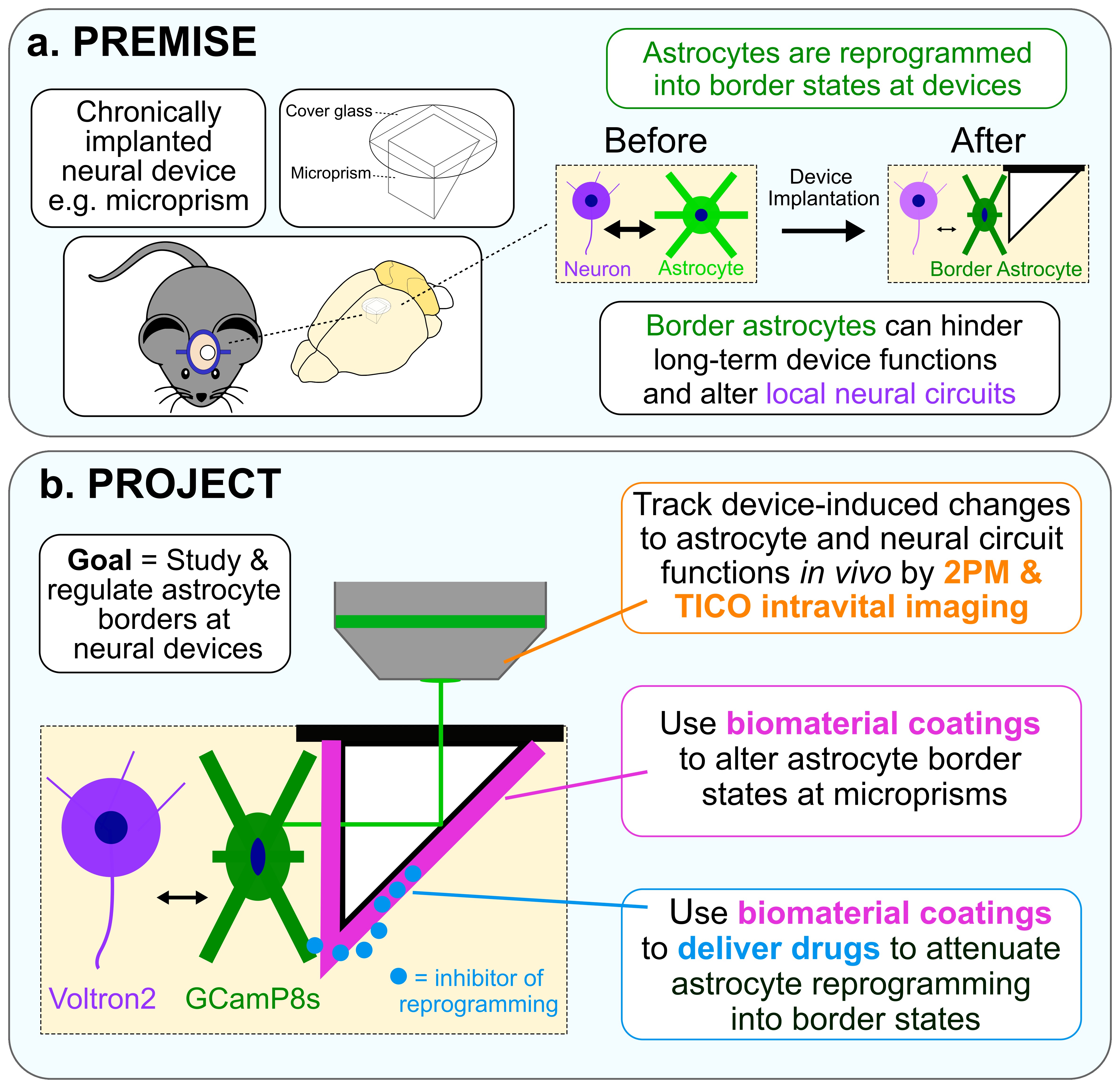
In this project we are using an implanted microprism system as a model chronically implanted neural device that can be used to provoke, functionally image, and regulate astrocyte borders. Microprisms are optical devices that can be used for longitudinal imaging of deep cortical regions in vivo using intravital microscopy methods including two-photon microscopy (2PM) and targeted illumination confocal (TICO). We are using microprisms to: (i) locally disrupt neural tissue and initiate astrocyte border transitions in a temporally precise and reproducible manner, (ii) track astrocytes and neurons labelled with functional fluorescent reporters during border formation using 2PM and TICO, and (iii) apply engineered interfaces to study how chemical, physical, and mechanical properties of devices alter border states.
Our main objectives for this project are to dissect astrocyte border formation and functions, test innovative biomaterial-based strategies to regulate astrocyte border biology, and understand how different astrocyte border states alter device-adjacent neural circuit activity. Using our newly developed cellulose-based coatings platform we are investigating how manipulating device surface properties and controllably delivering molecular regulators can be used to manipulate astrocyte responses to improve the viability and functionality of neural circuits at chronically implanted neural devices.
Why is this important?: The implantation of neuroprostheses and other biomaterial-based devices (e.g. local drug delivery systems or tissue engineering scaffolds) into the CNS initiates a foreign body response (FBR) that is unique to the CNS but mimics many characteristic features of the CNS wound response. The FBR to biomaterials in the CNS exists on a severity spectrum that is determined by definable biomaterial properties. We are beginning to understand how such biomaterial properties can be modified to minimize or evoke specific responses but much remains unstudied. A severe FBR can disrupt the long-term function of devices used in the CNS and represents a significant and current clinical problem across all neural interfacing fields. Since glia are principal actors in the orchestrated multicellular FBR to biomaterials we are targeting these cells for further study and manipulation.
3. Glia Engineering of Drug Delivery
Scientific Premise: We are beginning to understand that glial cell dysfunctions contribute to early-stage neural circuit damage that can precede clinical presentation of neurodegenerative diseases. Despite the contributions of glia to CNS disorder progression, we currently have limited approaches to target glial cells preferentially for therapy. The emergence of comprehensive glial cell specific transcriptomics information has enabled the identification of unique molecular features on the surface of different glial cells. The scientific premise guiding this research area is that cell specific transcriptomics information can be used to develop non-viral, polymer-based drug delivery vehicles with multivalent presentation of ligands that preferentially engage transporters or receptors uniquely expressed by different glial cell types to selectively target therapies to these cells.
Our Approach: Using our coacervate platform we are developing and testing non-viral strategies to deliver therapies, such as mRNA and small molecules, preferentially to specific glia that may be used to mitigate dysfunctional cellular activities or address mutations that are responsible for disease. Nano-sized coacervates are functionalized with carefully selected ligands to selectively interact with cell surface transporters that are uniquely expressed at high levels on microglia or astrocytes. As part of this research, we are applying targeted glia drug delivery approaches to rodent models of Parkinson’s disease (PD) and investigating how delivering therapies selectively to microglia can be used to correct dysfunctional phagocytotic functions in these cells that manifests in the early stages of PD.
Why is this important?: Glia dysfunction or mutations can lead to loss of healthy neural tissue function and cause disease. Identifiable glia dysfunction has been implicated in numerous CNS disorders including Huntington’s (HD), Parkinson’s, glioblastoma, neurodevelopmental and neurodegenerative diseases. Targeting drugs or gene therapies to dysfunctional glial cell populations in these CNS diseases in a clinically appropriate way will be important for furthering treatments. While viral vector-based delivery of therapeutics is used routinely in preclinical CNS models, numerous limitations of this technology exist. Additionally, various CNS tissue barriers present drug delivery challenges that remain to be addressed.
